Pharma and biomanufacturing organizations are facing a convergence of pressures: expiring patents, rising R&D costs, supply chain vulnerabilities, and growing expectations for speed and sustainability. At the same time, the complexity of therapies is escalating. The response? An urgent shift toward connected, digital-first systems that embed intelligence and resilience across the value chain. As development timelines tighten and regulatory demands intensify, organizations are reassessing the systems and processes that support innovation and production.
In this edition of Industry Signals, we explore three perspectives on how this transformation is taking shape:
- Deloitte offers a view into how modernized R&D labs can serve as strategic assets rather than cost centers;
- Siemens shares its vision for a digital value chain that connects lab, production, and compliance through technologies like simulation, digital twins, and AI; and
- BioPhorum provides an update on the state of MES in biomanufacturing, highlighting areas of progress and ongoing challenges as manufacturers work toward more modular, interoperable systems.
Together, they show that innovation is about the systems that bring science to life.
Deloitte on Modernizing the Pharma R&D Lab
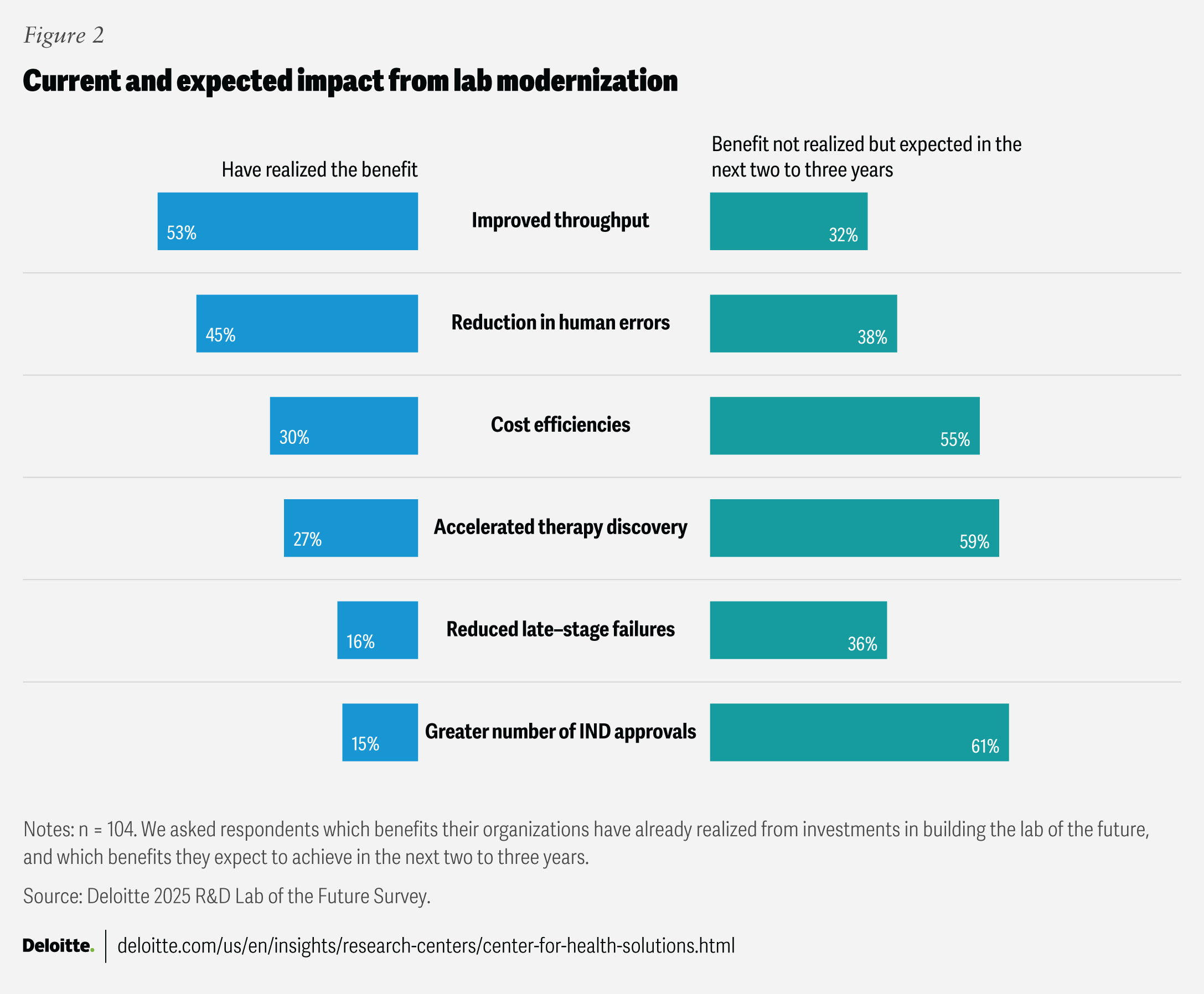
Source: Deloitte | Published: July 29, 2025 In Deloitte’s analysis of a recent survey of R&D executives, their Life Sciences and Health Care experts argue that biopharmaceutical organisations must urgently evolve their R&D labs into digital, automated, and data‑driven innovation engines to keep pace with mounting pipeline pressures, falling exclusivity and escalating costs. Key Points:
- Lab modernisation is linked to measurable performance gains: In the survey of 104 biopharma R&D executives, 53% reported increased laboratory throughput, 45% saw lower human error, 30% achieved better cost efficiency and 27% gained faster therapy discovery as a result of lab modernisation efforts.
- A strategic inflection point driven by the patent cliff and cost pressures: With patents on about 190 drugs expected to expire by 2030, and roughly US $236 billion in sales at risk, companies face a tipping point in needing internal innovation engines rather than outsourcing to fill gaps.
- Success requires a shift in data, operations and culture, and organizations should:
- Create a roadmap aligned with broader R&D strategy and measurable KPIs.
- Build a research‑data‑product mindset: connect instruments, integrate diverse data modalities, and make them reusable.
- Drive operational excellence and data governance: treat equipment as strategic assets, optimise workflow and ensure data integrity.
- Foster cultural change: equip scientists with digital skills and manage the change in working habits as much as the technology.
Accelerating MES Maturity in Biomanufacturing: Insights from BioPhorum
Source: Biophorm | Published May 30, 2025 In 2022, Biophorm released the MES of the Future manifesto, which addressed the gap between biomanufacturing needs and manufacturing execution system (MES) solutions. Recently, they shared a progress report update on this manifesto, which shows that service‑providers and manufacturers are increasingly aligning around modular architectures; data integration; and flexible deployments, but significant gaps remain, particularly in operations/support and initial deployment maturity. The update breaks down 120 distinct solution offerings to give biomanufacturing professionals clearer benchmarking and planning tools. Key Points:
- Modular architecture and integration/data‑access lead the pack: A survey of eight MES supply partners found the highest levels of progress in modular system structures and data‑access/integration capabilities. These areas, which are critical for agility, real‑time analytics and harmonising disparate systems, are advancing fastest.
- Operations & support and initial deployment lag behind: Although strategic alignment is improving, implementation in the more tactical domains of operations support, maintenance, and initial roll‑out remains uneven across suppliers. This highlights a critical risk for biomanufacturers aiming for scalable, resilient production platforms.
- For live‑therapeutics, the MES shift is now imperative: MES must become a flexible, data‑centric digital backbone of biomanufacturing. Investing in systems with modularity, cloud‑readiness, recipe reuse, and full life‑cycle support will be a differentiator as product pipelines move toward cell and gene therapies, continuous processing and personalized medicine.
Siemens’ Recipe for Accelerated Pharma Innovation
Source: Siemens | Published: June 4, 2025 In a recent thought leadership piece, Siemens discusses the idea that pharmaceutical and life‑sciences companies can drastically shorten development timelines by embracing a fully connected, digital value chain to leverage data, simulation, and seamless transfer from lab to production to bring therapies to patients faster while maintaining quality and compliance. Key Points:
- Digital threads as the backbone of pharma transformation: The concept of “Digital Threads” functions like a subway map guiding the full lifecycle from lab experiments, to pilot, to commercial manufacturing, ensuring knowledge and context travel with the product and nothing gets lost in hand‑offs.
- Simulation and digital twin tools accelerate design and development: Using simulation at the molecular level (tablet composition, particle size), and manufacturing process simulation (temperature, mixing, scale‑up) allows companies to reduce physical trials, speed development, and iterate more quickly.
- AI dramatically amplifies experimentation and learning: By applying artificial intelligence, simulations become smarter: past runs feed future predictions, enabling hundreds of experiments per hour and thereby accelerating development and reducing resource use (e.g., raw materials, energy).
- Becoming a ‘sustainable Digital Enterprise’ is critical to staying competitive: The goal is to re-architect the pharma value chain so that speed, flexibility, quality, compliance, and sustainability are built in.
It is also worth noting that at Hannover Messe 2025, Siemens showcased an integrated suite of digital‑and‑real‑world solutions illustrating how pharmaceutical and life‑sciences companies can compress drug development cycles, scale production intelligently, and embed sustainability into every step of their value chain. By combining modular labs, digital‑twin enabled manufacturing, and advanced materials with software and services, therapies can be brought to market faster, more reliably, and with less waste. You can read more about it here. Looking Ahead
While each organization’s path will differ, the direction of travel is consistent: toward more connected, digitally enabled ecosystems that support speed, reliability, and adaptability. Priorities include better data integration, flexible architectures, and alignment between R&D and production. For companies in life sciences and biomanufacturing, building this kind of resilience is essential.
That’s a wrap for this edition of Industry Signals. Have a report, use case, or event you'd like to see featured in an upcoming issue? Send a note via PM. We’re always looking to spotlight what’s shaping the future of industry and find recommendations from the Xcelerator Community especially valuable. Your insights and experiences continually shape Industry Signals.